Jamr F1701T BP monitor has passed clinical validation and meets the AAMI/ESH/ISO Universal Standard (ISO 81060-2:2018) for BP monitoring. The full clinical report was published online on National Library of Medicine on Mar 7, 2023, click the following link to check out the full report: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10132453/
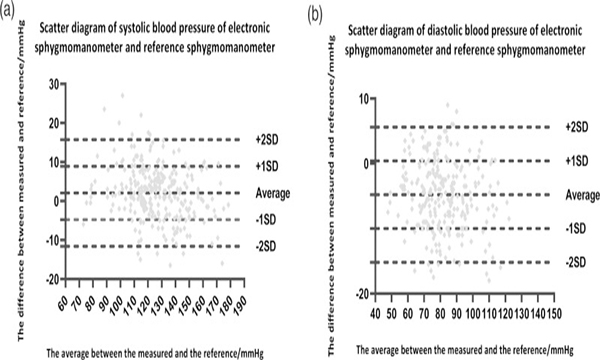
In the study, a total of 270 sets of comparison data (three sets of each subject) were obtained and analyzed. According to the validation criterion 1 of ISO 81060-2:2018, the mean ± SD of the differences between the Jamr F1701T and mercury sphygmomanometer BP (systolic/diastolic) readings was 2.06 ± 6.83/−4.84 ± 5.23 mmHg. For criterion 2, the SD of the averaged BP (systolic/diastolic) differences between the Jamr F1701 and reference BP (systolic/diastolic) per participant was 5.62/4.39 mmHg (the requirement was ≤6.43/5.01 mmHg by calculation).
This arm type monitor has excellent performance and is perfect for measuring blood pressure at home or in clinics.